dental line
Sus Mem

SUS-MEM is the family of reabsorbable membranes from TISSUM Biomaterials obtained from the processing of the pericardial sac of porcine origin.
The exclusive TISSUM TECH decellularization process allows to purify the collagenated matrix of the native tissue of the pericardium, obtaining a membrane free of antigenic compounds and preserving the integrity of the structure of the pericardium dense collagen fibers, thus obtaining a biomaterial which is: Sterile, Biocompatible, Hydrophilic, Occlusive, of High Mechanical Resistance, Suturable and Reabsorbable.
SUS-MEM membranes have an estimated reabsorption time of about 4 to 8 weeks after implantation.
Reabsorbable collagen membrane of porcine origin.
Composition/active substance:
Collagen of 100% porcine origin.
Presentations/models:
| Description | Size |
|---|---|
| Membranes | 1.5 x 2 cm |
| 2 x 3 cm | |
| Strands | 1.0 ml |
| 2.0 ml |
Structure composed of dense interlaced collagen strands which create a highly consistent and resistant three-dimensional mesh.
It offers the specialist surgeon the following properties:
Flexible product and easily adaptable to implant site.
Allows suturing of adjacent tissues.
Offers excellent barrier action and interface between membrane/graft and membrane/periosteum.
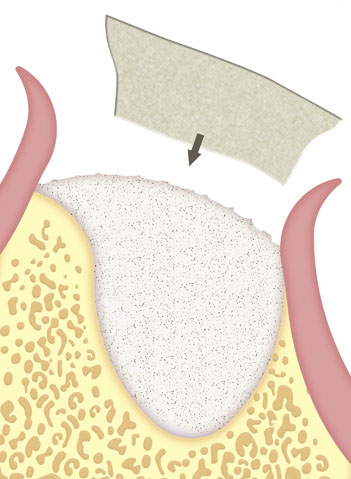
Provides a controlled reabsorption which protects graft placed at the implant site.
It is an occlusive material since it does not allow cell migration.
Contains and stabilizes the blood clot within the implant site.
Works as a barrier during the critical healing period.
CLINICAL INDICATIONS
Stomatology, maxillary, facial and implant surgery.
In periodontics, oral surgery and endodontics, to promote the regeneration of periodontal tissues.
Tissue regeneration in trauma, dermatology and ophthalmology.
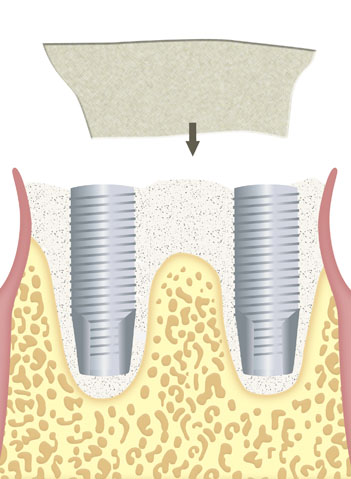
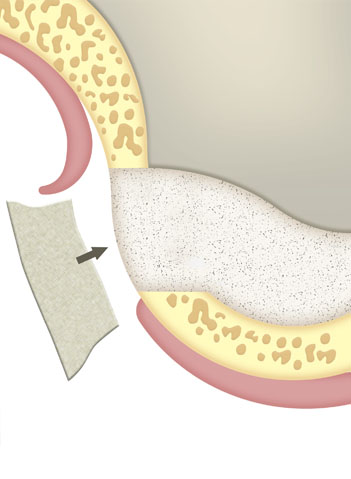
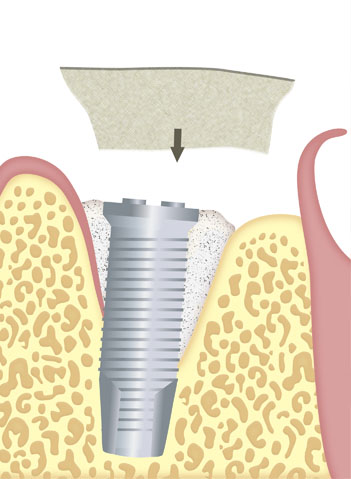
Immediate or delayed guided regeneration of tissues and bones after application of the membrane alone or in combination with appropriate augmentation materials (e.g. autologous bone material, allogeneic, xenogenic or alloplastic bone replacement materials).
Surgical bone defects and bone walls in the framework of an elevation of the maxillary sinus floor (sinus lift), or to reinforce the sinus mucosa (Schneider’s membrane).
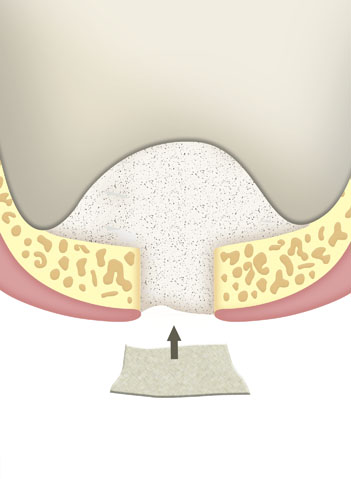
Within the framework of an augmentation of the alveolar process or a reconstruction thereof, carried out in order to facilitate the fixation of a dental prosthesis.
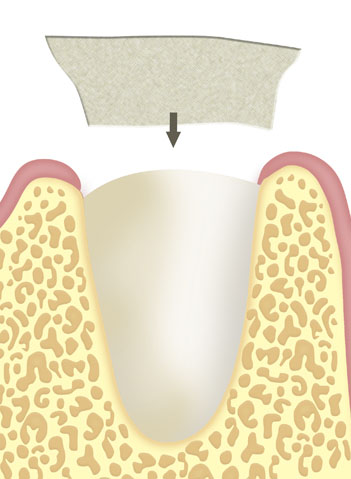
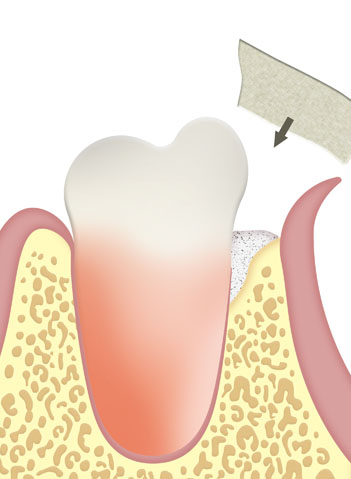
Periodontic bone defects, tissue dehiscence. After apicoectomies, cystectomies, extraction of retained teeth and resection of other bone defects.
In or next to the alveoli after tooth extraction.
As part of an immediate or delayed increase in dental sockets located around implants.
In neurosurgery, as a dura mater patch.
In orthopedics, when graft protection and/or soft/hard tissue neoformation is required.
For tissue regeneration in skin lesions. As a non-adhesive membrane between tendons. In tissue regeneration in hand, foot, knee, tibia or shoulder surgeries.
INSTRUCTIONS FOR USE
This membrane can be applied directly or after being hydrated with sterile saline solution or the patient’s own blood, for at least 15 minutes.
This material must be applied to the defect using sterile instruments.
It can be fixed with absorbable suture material and a blunt needle, or with fixation screws or pins.
Dentistry: to close the wound, the mucoperiosteal flap must be sutured on the membrane in a tight manner, without tensioning it. If possible, close the wound completely by covering the membrane with the mucoperiosteum flap.
The dentist will recommend the applicable oral hygiene measures.
Postoperative monitoring is recommended.
The described technique should only be carried out in an appropriate environment and with sterile instruments.
ADVERTENCIAS Y PRECAUCIONES
Exposure of this product during the healing phase may cause accelerated absorption.
This product should only be used for its intended use.
Not intended for patients with particularly severe surgical, implant, endodontic or periodontal defects.
The physician must warn the patient of the need to return to the consultation in the event of any type of postoperative disorder, such as pain, infection and other abnormal symptoms.
The patient should be advised of contraindications, warnings and precautions.
Special care should be taken in patients with serious illnesses (e.g. diabetes mellitus, uncontrolled hypertension, corticosteroid therapy, treatment with oral antiplatelet and anticoagulant medication, autoimmunopathies, malignancies, etc.)
The INSTRUCTIONS FOR USE included with the product must be read carefully by the dentist and must be informed to the patient.
In exceptional cases, symptoms of intolerance and/or an inflammatory reaction of the tissue may occur.
Allergic reactions to the collagen membrane cannot be ruled out in isolated cases.