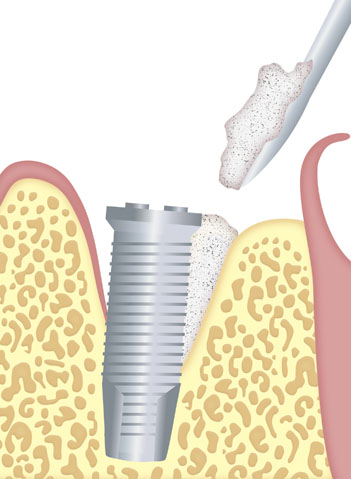
Dental line
Sus oss
SUS-OSS is the family of collagenated dental bone fillings from TISSUM BIOMATERIALES.
Produced under the exclusive TISSUM TECH decellularization process, in which it is possible to purify the native bone tissue of porcine origin, through low temperature chemical processes, preserving the native structure of collagen and hydroxyapatite, being a xenograft with characteristics similar to bone tissue of human origin. SUS-OSS biomaterials come in different formats and presentations: granules, blocks, tables and sheets.
Among the distinctive characteristics of SUS-OSS products we can mention: Sterile, Biocompatible, Osteoconductive and with the presence of native collagen.
Generic name:
Extracellular bone matrix of porcine origin for guided tissue regeneration
Composition:
Bone matrix composed of hydroxyapatite and collagen of porcine origin.
Presentations:
| Code | Particle size |
|---|---|
| F | < 210 µm |
| N | 210 - 1000 µm |
| G | 1,000 - 2,000 µm |
| EG | > 2,000 µm |
FEATURES
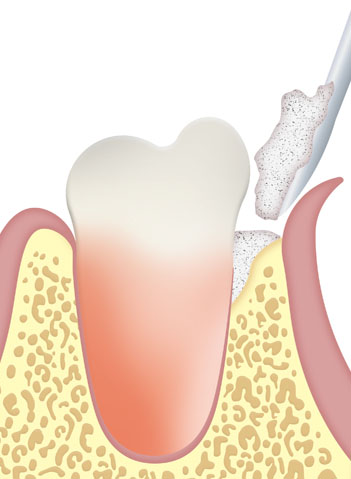
With a high osteoconductive capacity and thanks to the presence of collagen, it also has osteoinductive capacity, which allows the repair and growth of tissue in the implanted area.
It offers the specialist surgeon the following properties:
Sterile, inert and biocompatible material.
Hydrophilic and easy to adapt to the implant site.
CLINICAL INDICATIONS
Bone substitute in medical surgery.
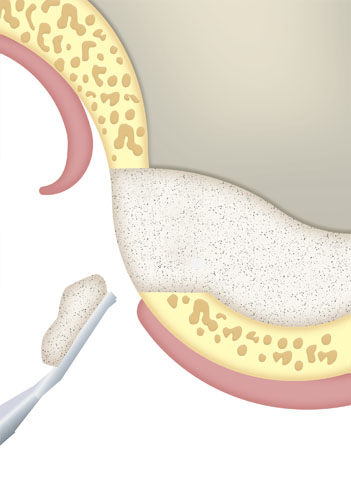
Loss of bone substance, need for augmentation and filling of cavities that must be covered with bone tissue.
Filling of bone cavities in traumatology, dentistry and ophthalmology.
Bone reconstruction and regeneration in traumatology, dentistry and ophthalmology.
Bone reconstruction and regeneration in spinal surgery.
Filler in ocular evisceration and enucleation.
Filler material in the eye socket.
Cranioplasties.
Maxillofacial surgery.
Other clinical indications
INSTRUCTIONS FOR USE
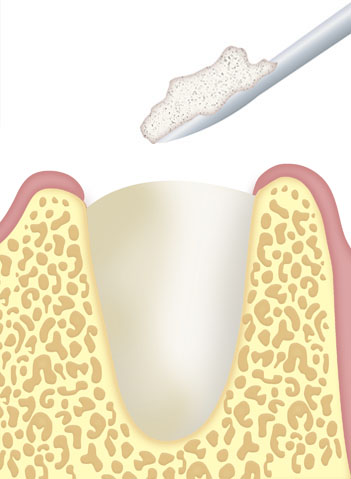
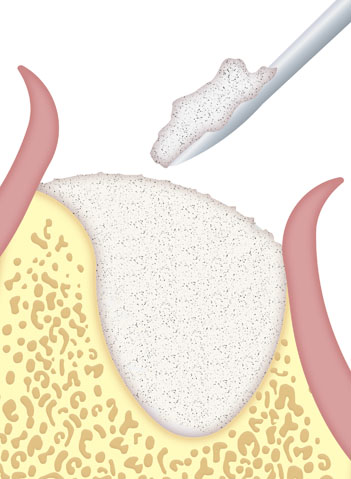
Apply the material to the defect using sterile instruments.
Ensure optimal contact between the bone filling and the recipient bone in order to ensure correct osteoinduction.
Modulate with spatula, if necessary.
Avoid excess product in the defect.
Place a membrane to protect the soft tissue graft, in cases where this type of protection is required.
The implant specialist must take into account the strength of the bone filling when loading it and, where appropriate, placing a support structure.
Ensure the correct immobilization of the filling in order to avoid micro movements that could generate a fibrous encapsulation.
The surgical technique described must be carried out exclusively in an appropriate environment and with sterile instruments.
WARNINGS AND PRECAUTIONS
To facilitate bone neoformation, the implanted material must be in direct contact with bone walls with good vascularization and, in certain cases, it is recommended to prepare the patient’s bone tissue with a bur.
In the case of large cavities, a mixture of this biomaterial with autologous bone can improve neoformation.
Implantology (Dentistry): Experience shows that in areas where bone mass has increased, mechanical load or the definitive insertion of implants should not be applied until at least 4 to 6 months have elapsed from the insertion of the material.
Periodontics (Dentistry): Before applying the biomaterial, it is necessary to perform a correct treatment of the periodontal lesion by root scaling, curettage and other appropriate procedures.
Discard any remnants and do not attempt to resterilize the biomaterial. Do not use after the expiration date.