dental line
Sus Mem
SUS-MEM is the family of reabsorbable membranes from TISSUM Biomaterials obtained from the processing of the pericardial sac of porcine origin.
The exclusive TISSUM TECH decellularization process allows to purify the collagenated matrix of the native tissue of the pericardium, obtaining a membrane free of antigenic compounds and preserving the integrity of the structure of the pericardium dense collagen fibers, thus obtaining a biomaterial which is: Sterile, Biocompatible, Hydrophilic, Occlusive, of High Mechanical Resistance, Suturable and Reabsorbable.
SUS-MEM membranes have an estimated reabsorption time of about 4 to 8 weeks after implantation.
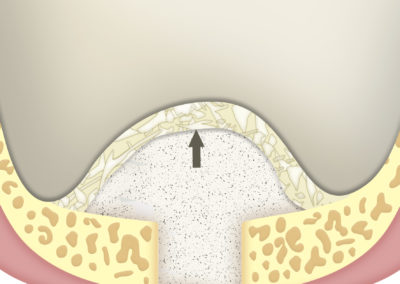
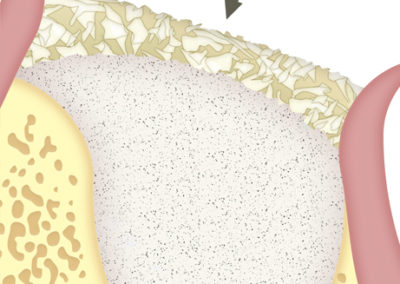
Collagen strands.
Composition:
Type I and III collagen of porcine origin.
Presentations:
| Description | Size |
|---|---|
| Collagen strands | 1 ml |
| Collagen strands | 2 ml |
2 months approx.; depending on the characteristics of the implant site and the patient’s health conditions.
Hemostatic action
Reabsorbable
Biocompatible
Adaptable to the implant site
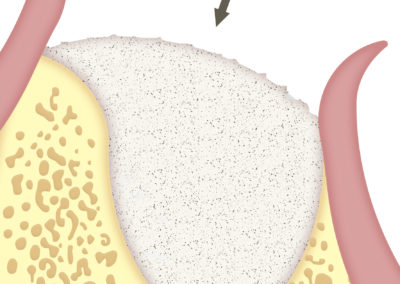
SUS-MEM TISSUM® STRANDS is a biomaterial made of collagen in strands, and MAY be used alone or as a complement to hydroxyapatite, in which case it provides the benefits of native collagen: hemostasis, osteoinduction and clot stability. The strand format allows a homogeneous mixture that, once hydrated, facilitates the handling of the filling, acting as a binder to transfer it more easily to the implant site. In addition, it shortens the regeneration times.
When used alone, it is recommended for soft tissues, as it improves the aesthetic appearance of surgeries.
SUS-MEM TISSUM® STRANDS is indicated in the following cases:
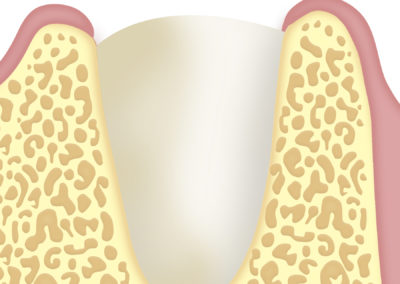
Implantology: to cover and protect bone grafts.
Use as a barrier in tissue regeneration.
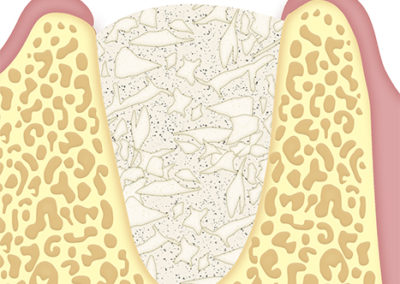
The application of the strands in combination with bone substitutes is indicated for guided tissue regeneration.
In maxillary sinus lift or to reinforce the sinus mucosa.
In the augmentation and reconstruction of the alveolar process in order to facilitate the fixation of a dental prosthesis.
Other medical indications.
SUS-MEM TISSUM® STRANDS is used as an interface to safely lift the membrane of the maxillary sinus, either by the transalveolar surgical procedure or through a window. In both cases, by placing them as an interface to later push the sinus membrane, a safer maneuver is performed, minimizing the risks of occasional injury. Its use will act as a biomaterial for regeneration, in particular, if there is a small lesion in the sinus membrane.
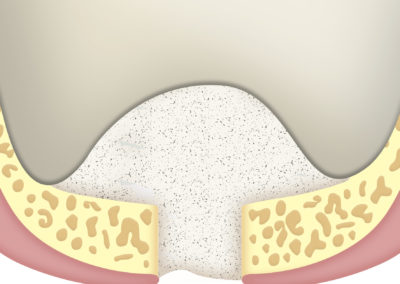
As a barrier between bone filling and soft tissues:
Due to its high hemostatic power, the application of a layer of SUS-MEM TISSUM® STRANDS on the bone filling applied on the site of the defect provides a biological barrier that prevents the invasion of soft tissues, protecting the implant site.
Due to their ease of use, it is not necessary to cut them to the shape to be covered.
They are used to cover the bone substitute of the sockets in which flaps are not made.
They can also be used in post-extraction cavities of cysts or tumors
SUS-MEM TISSUM® STRANDS can be applied directly, or after previous hydration with a sterile saline.
Close the wound, in the clinical cases that are required. If possible, close it completely by covering the membrane with the mucoperiosteal flap.
The dentist will recommend the pertinent oral hygiene measures. Postoperative monitoring is recommended.
IMPORTANT: this product must be used by professionals specialized in biomaterials. It should never be applied without careful planning prior to surgery.
Exposure of this product during the healing phase can cause accelerated absorption.
This product is to be used only for its intended use.
The professional must inform the patient that in the event of any type of post-operative disorder (pain, infection or other abnormal symptoms) they should immediately consult a doctor.
The professional should warn the patient about the contraindications, warnings and precautions of this product.
Special care must be taken in patients with serious illnesses. As with any material foreign to the body, previous infections could be worsened. The INSTRUCTIONS FOR USE that are included with the product must be read carefully by the professional and must be made known to the patient.
Not intended for patients with particularly severe surgical, implant, endodontic or periodontal defects.